18+ Calculate Delta G For The Reaction At 298 K
The value of K_p at 298 K for the reaction frac12 N _2frac32 H _2 rightleftharpoons 2 NH _3 is found to be 8260 partial pressures being measured in atmospheric units. Delta G circ ΔG.
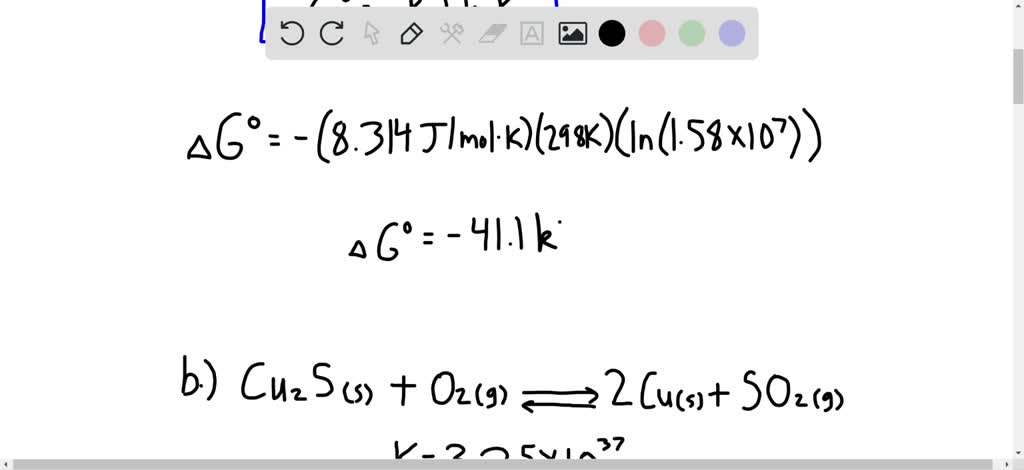
Solved Calculate Dg At 298 K For Each Reaction A 2 No G Cl2 G 2 Nocl G K 1 58 10 7 B Cu2s S O2 G 2 Cu S So2 G K 3 25 10 37
2Als3Cu2aqrarr2Al3aq3Cus Given E_cell202 V.
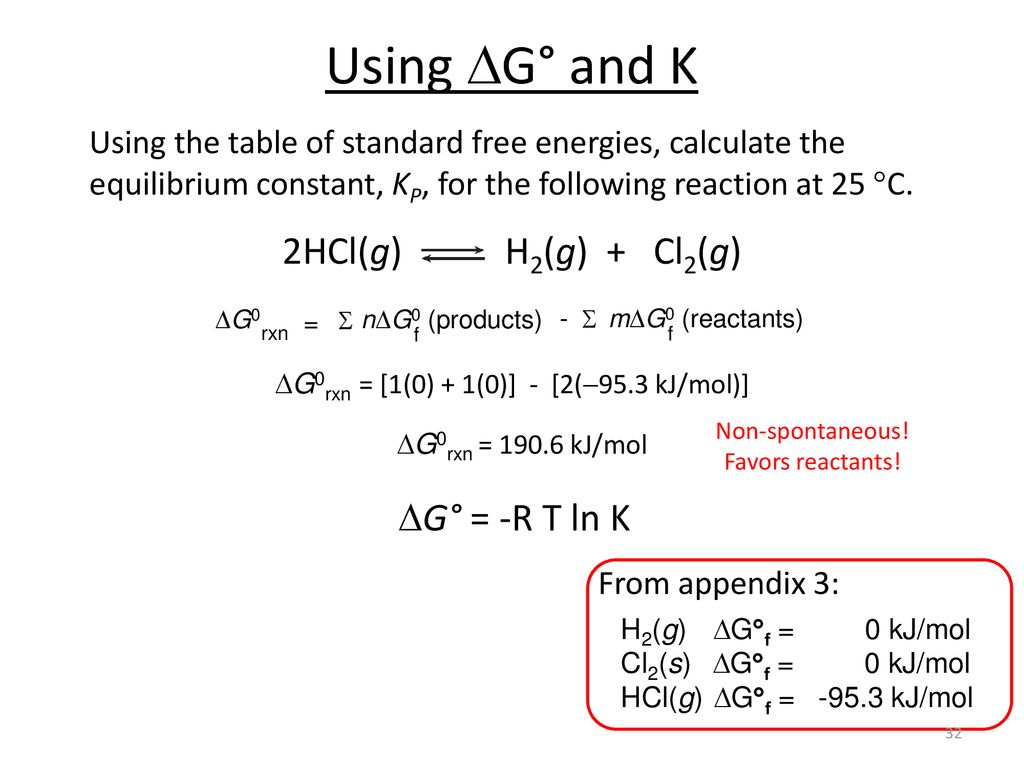
. Find step-by-step Chemistry solutions and your answer to the following textbook question. Calculate Delta G_mathrmrxncirc at 298 mathrmK for the following reaction. Calculate delta G at 298 K.
ASK AN EXPERT Science Chemistry QA Library Calculate delta G at 298 K. See Answer Calculate Δ𝐺ΔG at 298 K for the reaction CS2 l3O2 g CO2 g2SO2 gCS2 l3O2 g CO2 g2SO2 g based on these reactions. Calculate the free energy change at 298 K for the reaction Br_2l Cl_2g rarr 2BrClg.
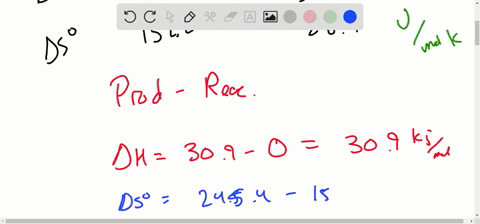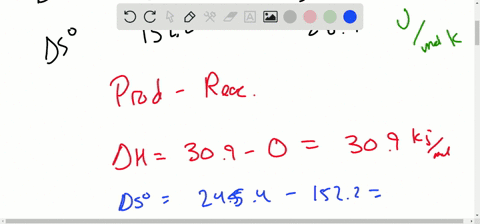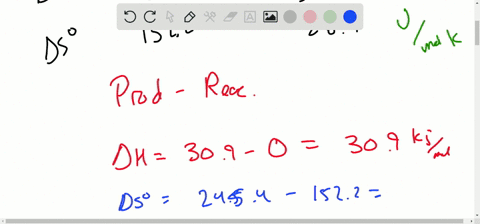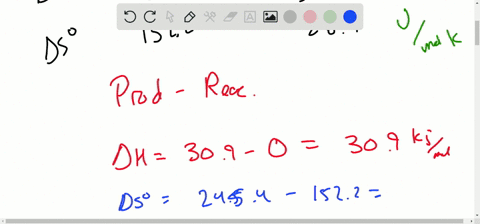
ΔG ΔG RT ln Q with Q the reaction quatient with T the. Delta H circ ΔH. Delta S circ ΔS.
From the values given for. For the reaction DeltaH293 KJ the entropies of B. Question thumb_up 100 An oxidation reduction reaction has 3 moles of.
Eight point 314 Jules for more times Kelvin times a temperature of. C sO2 g CO2 g Δ𝐺39728. Calculate Delta Gcirc at 298 K for these reactions and predict the effect on Delta.
Calculate DeltaG and log K_c for the following reaction at 298 K. For each of the following reactions at 298 K. Data given ΔG NO2 5184 kJmol ΔG N2O4 9828 kJmol Step 2.
Calculate G for the reaction at 298 K 2SO 2gO 2g 2SO 3g G o142kJmol 1 If partial pressures of SO 2 O 2 and SO 3 are 50 atm 75 atm and 2 atm respectively at 298K Medium. M2Os 2Ms 12O2g Substance delta G. If you want to calculate ΔG under non-standard conditions you need to use the equation ΔG ΔG0 RT lnQ where Q is the ratio of concentrations or activities of the.
Up to 256 cash back Consider the decomposition of a metal oxide to its elements where M represents a generic metal. For Delta G again the changing Gibbs free energy at standard conditions for this reaction is equal to negative or again.
Browse Questions For Chemistry 101

Solved Calculate Delta G At 298 K For The Reaction 2 N2o5 G Chegg Com

For The Reaction At 298 K A G B G Harrd G C G Deltah 29 8 Kcal And Deltas 100calk 1 Calculate The Value Of Equilibrium Constant

The Standard Reduction Potentials For Two Reactions Are Given Below Agcl S E Ag S Cl Aq E 0 22v Ag Aq E Ag S E 0 80v The

Gibbs Free Energy Temperature And Spontaneity Ppt Download

The Value Of D G For The Reaction At 298 K Is
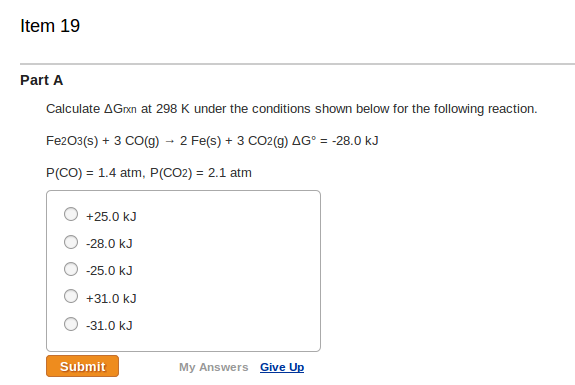
Solved Part A Calculate Delta Grxn At 298 K Under The Chegg Com
For The Reaction At 298 K A G B G Harrd G C G Deltah 29 8 Kcal And Deltas 100calk 1 Calculate The Value Of Equilibrium Constant

Innovative Chemistry Revision Notes Chem184 Innovative Chemistry For Energy And Materials Liverpool Thinkswap
Solved Calculate Dg At 298 K For Each Reaction A 2 No G Cl2 G 2 Nocl G K 1 58 10 7 B Cu2s S O2 G 2 Cu S So2 G K 3 25 10 37
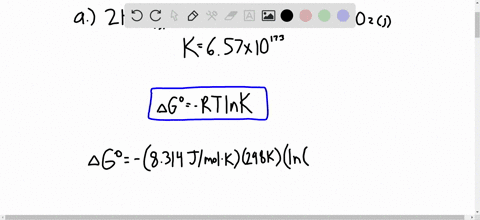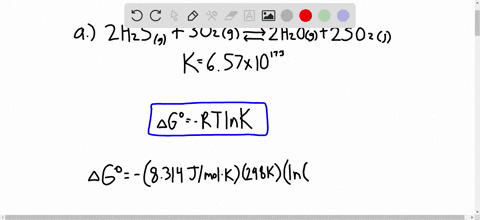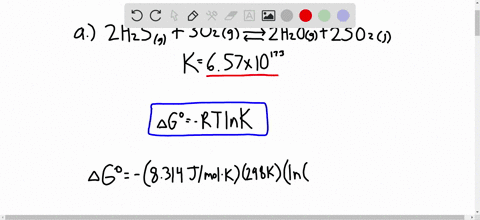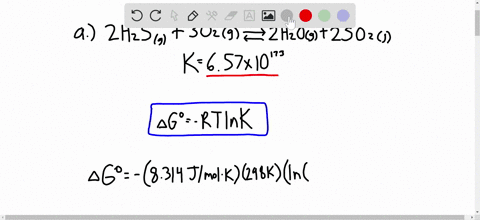
Solved Calculate Dg At 298 K For Each Reaction A 2 No G Cl2 G 2 Nocl G K 1 58 10 7 B Cu2s S O2 G 2 Cu S So2 G K 3 25 10 37

Math Physics Chemistry Questions Discussion Lists Dated 2018 07 16

Pdf Advices For Studying Organic Chemistry Sabrina Islam Academia Edu
First Document Gsi

The Gibbs Free Energy Post 16 Thermodynamics Tutorials Resource Rsc Education
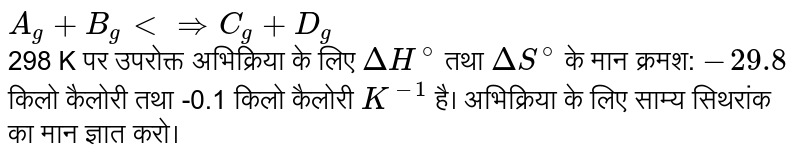
For The Reaction At 298 K A G B G Harrd G C G Deltah 29 8 Kcal And Deltas 100calk 1 Calculate The Value Of Equilibrium Constant

Cambridge Ibdp Chemistry Book Pdf Mole Unit Mixture